|
Cheap, plastic
solar cells may be on the horizon, thanks to new technology developed
by UC Berkeley, LBNL chemists
28 March 2002
By Bob Sanders, Media
Relations
Berkeley - University
of California, Berkeley, chemists have found a way to make cheap plastic
solar cells flexible enough to paint onto any surface and potentially
able to provide electricity for wearable electronics or other low-power
devices.
The group's first
crude solar cells have achieved efficiencies of 1.7 percent, far less
than the 10 percent efficiencies of today's standard commercial photovoltaics.
The best solar cells, which are very expensive semiconductor laminates,
convert, at most, 35 percent of the sun's energy into electricity.
 |

A
panel of eight plastic solar cells based on inorganic nanorods and
semiconducting polymers. The shiny ovals are the aluminum back electrodes
of the individual solar cells.
|
"Our efficiency is
not good enough yet by about a factor of 10, but this technology has
the potential to do a lot better," said A. Paul Alivisatos, professor
of chemistry at UC Berkeley and a member of the Materials Science Division
of Lawrence Berkeley National Laboratory. "There is a pretty clear path
for us to take to make this perform much better."
Alivisatos and his
co-authors, graduate student Wendy U. Huynh and post-doctoral fellow
Janke J. Dittmer, report their development in the March 29 issue of Science.
"The beauty of this
is that you could put solar cells directly on plastic, which has unlimited
flexibility," Dittmer said. "This opens up all sorts of new applications,
like putting solar cells on clothing to power LEDs, radios or small computer
processors."
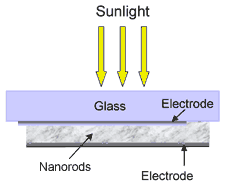
The solar cell they
have created is actually a hybrid, comprised of tiny nanorods dispersed
in an organic polymer or plastic. A layer only 200 nanometers thick is
sandwiched between electrodes, and can produce, at present, about 0.7
volts. The electrode layers and nanorod/polymer layers could be applied
in separate coats, making production fairly easy. And unlike today's
semiconductor-based photovoltaic devices, plastic solar cells can be
manufactured in solution in a beaker without the need for clean rooms
or vacuum chambers.
"Today's high-efficiency
solar cells require very sophisticated processing inside a clean room
and complex engineering to make the semiconductor sandwiches," Alivisatos
said. "And because they are baked inside a vacuum chamber, they have
to be made relatively small."
The team's process
for making hybrid plastic solar cells involves none of this.
"We use a much dirtier
process that makes it cheap," Huynh said.
The technology takes
advantage of recent advances in nanotechnology, specifically the production
of nanocrystals and nanorods pioneered by Alivisatos and his laboratory
colleagues. These are chemically pure clusters of from 100 to 100,000
atoms with dimensions on the order of a nanometer, or a billionth of
a meter. Because of their small size, they exhibit unusual and interesting
properties governed by quantum mechanics, such as the absorption of different
colors of light depending upon their size.
 |
Schematic
diagram of a hybrid "plastic" solar cell with a nanorod/polymer layer
sandwiched between two electrodes. The middle layer, a mere 200 nanometers
thick, is a jumble of nanorods embedded in the semiconducting polymer.
|
It was only two years
ago that a UC Berkeley team led by Alivisatos found a way to make nanorods
of a reliable size out of cadmium selenide, a semiconducting material.
Conventional semiconductor solar cells are made of polycrystalline silicon
or, in the case of the highest efficiency ones, crystalline gallium arsenide.
Huynh and Dittmer
manufactured nanorods in a beaker containing cadmium selenide, aiming
for rods of a diameter - 7 nanometers - to absorb as much sunlight as
possible. They also aimed for nanorods as long as possible - in this
case, 60 nanometers. They then mixed the nanorods with a plastic semiconductor,
called P3HT - poly-(3-hexylthiophene) - and coated a transparent electrode
with the mixture. The thickness, 200 nanometers - a thousandth the thickness
of a human hair - is a factor of 10 less than the micron-thickness of
semiconductor solar cells. An aluminum coating acting as the back electrode
completed the device.
The nanorods act
like wires. When they absorb light of a specific wavelength, they generate
an electron plus an electron hole - a vacancy in the crystal that moves
around just like an electron. The electron travels the length of the
rod until it is collected by the aluminum electrode. The hole is transferred
to the plastic, which is known as a hole-carrier, and conveyed to the
electrode, creating a current.
P3HT and similar
plastic semiconductors currently are a hot area of research in solar
cell technology, but by themselves these plastics are lucky to achieve
light-conversion efficiencies of several percent.
"All solar cells
using plastic semiconductors have been stuck at two percent efficiency,
but we have that much at the beginning of our research," Huynh said.
"I think we can do so much better than plastic electronics."
"The advantage of
hybrid materials consisting of inorganic semiconductors and organic polymers
is that potentially you get the best of both worlds," Dittmer added.
"Inorganic semiconductors offer excellent, well-established electronic
properties and they are very well suited as solar cell materials. Polymers
offer the advantage of solution processing at room temperature, which
is cheaper and allows for using fully flexible substrates, such as plastics."
Visiting scientist
Keith Barnham, professor of physics at Imperial College, London, and
an expert on high-efficiency solar cells, agreed.
"This is exciting,
cheap technology if they can get the efficiency up to 10 percent, which
I think they will, in time," Barnham said. "Paul's approach is a very
promising way to get around the problem of the efficiency of plastic
solar cells."
Some of the obvious
improvements include better light collection and concentration, which
already are employed in commercial solar cells. But Alivisatos and his
colleagues hope to make significant improvements in the plastic/nanorod
mix, too, ideally packing the nanorods closer together, perpendicular
to the electrodes, using minimal polymer, or even none - the nanorods
would transfer their electrons more directly to the electrode. In their
first-generation solar cells, the nanorods are jumbled up in the polymer,
leading to losses of current via electron-hole recombination and thus
lower efficiency.
They also hope to
tune the nanorods to absorb different colors to span the spectrum of
sunlight. An eventual solar cell might have three layers, each made of
nanorods that absorb at different wavelengths.
"For this to really
find widespread use, we will have to get up to around 10 percent efficiency,"
Alivisatos said. "But we think it's very doable."
The work was funded
by the National Renewable Energy Laboratory and the Department of Energy. |