|
Randy Schekman: A passion for yeast
 |

Randy
W. Schekman, professor of molecular and cell biology at
UC Berkeley and an investigator in the Howard Hughes Medical
Institute |
Randy Schekman's passion for science began in a southern California
junior high school, where he first saw older students creating
science fair projects. He was hooked.
"Starting in 8th grade and all the way through high school,
that was my one outlet. The whole year was geared around doing
some project for display," said Schekman, 53, professor
of molecular and cell biology and a member of UC Berkeley's
Health Sciences Initiative — a group of several hundred
researchers from many disciplines working together to advance
health care in the 21st century.
Born in St. Paul, Minn., in 1948, Schekman moved with his family
in 1960 to a suburban development called Rossmoor in Orange
Co., where he attended Western High School in Anaheim, the school
from which golfer Tiger Woods would one day graduate.
Nurtured along by his biology teacher, he accumulated medals,
trophies and even money at school and regional competitions
and at the California State Science Fair, to which he advanced
three times in high school. Upon graduation in 1966, he was
one of a mere dozen or so students in his class of 650 to go
on to college. His destination was UCLA, far enough away that
his mother was afraid the family couldn't afford his commute.
He enrolled anyway, as a premed student.
Exposure to research in his freshman year confirmed his passion
for science. It also gave him the guidance he needed, as did
James Watson, discoverer of the structure of DNA.
"Watson's book, called 'Molecular Biology of the Gene,'
was my bible in my freshman year," he said. "One of
the really amazing experiences I had in my sophomore year was
(reading) Watson's autobiography, 'The Double Helix.' When it
came out in 1967, it was serialized in the Atlantic Monthly,
and I remember running to the library to pick up copies of the
magazine to read it all night. Not that I ever expected to make
discoveries like that or to act like that — but that one
could actually plumb the depths of nature with intellect and
intuition and work was a revelation."
He got a true taste of cutting-edge scientific research in
a UCLA lab where he studied DNA replication, that is, how genetic
material is copied in preparation for cell division. This interest
led him to shoot for the top —graduate work in the molecular
biology hotbed at Stanford University and the lab of renowned
biochemist Arthur Kornberg.
"Kornberg was the master of DNA replication, and I decided
that was what I really needed to do, go learn biochemistry from
the master," Schekman said.
He was successful, moving north in 1970 to a whole new world.
"The ferment in that department was unbelievable –
for DNA work, it was the center of the universe," he said.
While Schekman was purifying enzymes involved in chromosome
replication, Stanford professor Paul Berg in 1972 created the
first recombinant DNA by snipping and recombining segments from
two different viruses. A year later, Stanford colleague Stanley
Cohen teamed up with UC San Francisco professor Herbert Boyer
to insert recombinant DNA into bacteria that could be cloned
— a discovery that launched the genetic engineering revolution
and the first company to exploit the technology to make pharmaceuticals,
Genentech.
"I learned a great deal, not just about biochemistry or
DNA, but how to look at a problem, how to take it apart and
put it back together, how to focus on things," said Schekman.
"It really set the stage for me. Focus, in particular,
was critical to my work, and I learned that from Kornberg."
But the atmosphere in Kornberg's lab was highly competitive
and the field of DNA replication contentious. So, Schekman found
a less competitive niche at UC San Diego, where he spent two
postdoctoral years with John Singer studying cell membranes.
Singer was doing groundbreaking work with electron microscopy
to study the membranes or outer shells of mammal cells, but
Schekman was frustrated at the lack of tools to manipulate these
cells. Used to the many tools available to study bacteria, he
decided to focus on another easy-to-grow microbe, baker's yeast,
in hopes that combining genetics and biochemistry would lead
to an understanding of the machinery of secretion.
Thrilled to be offered a position as assistant professor of
biochemistry at UC Berkeley in 1976, he left bacteria and mammalian
cells behind to focus on yeast genetics. Unfortunately, while
mammal cells bustle with transport vesicles which are easy to
see with a microscope, yeast seemed to have less activity that
was harder to see. It's well known that yeast exude alcohol
through their membranes, but it wasn't clear how they secrete
proteins.
Given Schekman's lack of experience with yeast and the question
of whether secretion in yeast would apply to humans, his first
grant application was turned down. But Schekman had faith, and
has since proven the early doubters wrong.
 |
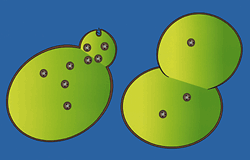
Yeast
cells grow a bud as protein-filled vesicles shuttle to the
surface and merge with the cell membrane, at the same time
releasing their cargo of protein. |
The key insight by Schekman and graduate student Peter Novick,
now a professor at Yale University, was that the best way to
track down genes involved in the secretion pathway was to look
for mutant yeast that die, since the pathway is critical to
the life of the cell. To find strains to raise and study, they
looked for those that survived at low temperatures but died
at higher temperatures, around body temperature.
"When I first came here, I had no plans to be a geneticist.
I was a biochemist," said Schekman. "But I found it
was the most tractable way to begin. Define the process using
genetics, then use the genetics to bootstrap into the molecular
work. That was the game plan."
With this approach, he eventually found and identified nearly
50 genes involved in the secretion pathway of yeast. These govern
every step from the formation of vesicles and selection of proteins
in the endoplasmic reticulum — an internal "corral"
for chemicals the cell doesn't want to mix with the rest of
the cell's contents — to their transport along the rail
system of the cell to the Golgi and then to the cell surface,
expelling proteins and growing the membrane so the cell eventually
can bud daughter cells. The work of Schekman and Rothman, who
was looking for proteins involved in secretion by mammalian
cells, soon began to complement one another, and it became clear
that yeast and humans secrete proteins using much the same machinery.
 |
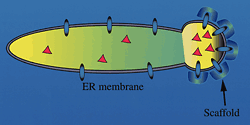
Proteins
form a scaffold around a section of the cell's endoplasmic
reticulum, recruiting cargo proteins (red triangles) destined
for the cell surface. The scaffold protein complex, called
COPII, warps the membrane surface until it pinches off into
a vesicle. |
One important finding was that a complex of three proteins
must glom onto the surface of the endoplasmic reticulum before
the membrane can bud to form transport vesicles. Thousands of
these complexes, dubbed COPII, coat the budding surface, deforming
it into a sac and also recruiting the correct protein cargo.
Schekman and his UC Berkeley students proved this by creating
artificial membranes, liposomes, in a test tube and coating
them with COPII. Given an energy boost, the liposomes budded
"with the same high efficiency and fidelity we have with
yeast," he said.
Recent findings about Alzheimer's disease hint that some patients
may have a problem with this secretion pathway. A protein called
amyloid precursor protein is normally rounded up in the endoplasmic
reticulum and packaged for transport to the cell surface. A
hitch in the machinery makes the protein susceptible to an enzyme
that clips it to produce amyloid, a nonbiodegradable protein
that accumulates until the cell is so overloaded it dies. Schekman
is investigating this lead in hopes of finding a way to prevent
amyloid production.
Interestingly, a rare form of hemophilia — factor-5, factor-8
deficiency — may also result from a defect in the packaging
machinery. Patients with this form of the disease have low levels
of two different clotting factors, making a simple cut a life-threatening
bleed. In 70 percent of these patients, the clotting factor
membrane receptor that COPII binds to is defective and interferes
with the secretion of these two clotting factors.
Even Type II diabetes, once known as adult-onset diabetes,
can involve problems with secretion. Normally, insulin stimulates
cells to take in glucose through specialized glucose channels
that, like all cell surface proteins, are transported to the
surface aboard membrane vesicles. Some patients don't respond
to insulin because they have too few glucose channels, and the
problem may be a bottleneck in the packaging of channels into
vesicles for their trip to the surface. The challenge, Schekman
said, is to find a therapeutic intervention that will bypass
the normal process and stimulate the discharge of the internal
reservoir of glucose channels so the cell can respond normally.
Since mobilization of glucose channels by insulin is hard to
study in human cells, Schekman has begun to study regulated
transport in yeast instead. The model he is looking at involves
how stress triggers the release of enzymes that make the cell
wall more rigid, using the same molecule, chitin, that insects
have adapted for their hard exoskeletons.
Schekman admits that his once isolated research niche is now
crowded, in part because his research opened up many new avenues
for exploration. Although he can't pursue them all, he's grateful
many of his students can.
His children won't be following that path, though. Both are
musically inclined, reflecting Schekman and his wife, Nancy
Walls', interest in classical music and opera. Joel is now a
graduate student at the University of Southern California, a
classical musician studying clarinet. Schekman's daughter, Lauren,
is a junior majoring in economics at UC Berkeley.
"I've had a wonderful time here at Cal, but the high point
of my 27 years was when she was admitted as a student. Even
more so, when I had the opportunity as a proud father to tour
the campus with her on Cal Day 2000. I was in 7th heaven."
He looks back with nostalgia at the trials he's weathered at
UC Berkeley, among them the cramped lab space in deteriorating
Barker Hall and his current relocation to Stanley Hall, itself
slated for demolition.
"That's why I love this place. You come here and have
to fight for what you get," he said. "That's the real
world, and that's why I think this place is such a valuable
experience. If you can make it here, you can make it anywhere."
—
Robert Sanders
Additional
information:
|