Immune therapy shows potential against melanoma, ovarian cancer
BERKELEY – An immune therapy discovered at the University of California, Berkeley, can boost the benefit of some cancer vaccines, according to a small, preliminary study led by researchers at Dana-Farber Cancer Institute and Brigham and Women's Hospital in Boston.
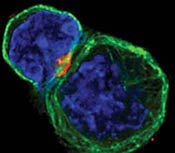 An immune system T cell, left, in contact with an antigen-presenting cell, which primes the T cell to seek out and destroy an invader. A receptor called CTLA-4, in red, is now at the immunological synapse between the cells. Blocking CTLA-4 with specially made antibodies unleashes the killing power of T cells against cancer cells. (Allison lab, UC Berkeley) 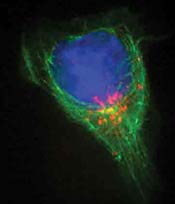 An activated T cell crawling around looking for invading cancer cells. A receptor called CTLA-4 is in red, inside the cell and ready to move. Blocking CTLA-4 with specially made antibodies unleashes the killing power of T cells against cancer cells. (Allison lab, UC Berkeley) |
The experimental therapy blocks a key protein on immune system T cells, releasing a natural brake on the immune system and unleashing an assault on the cancer.
The study, published online last week in the early edition of the Proceedings of the National Academy of Sciences, tested the effect of a single injection of an antibody, MDX-CTLA4, in nine patients who had previously been treated with cancer vaccines for either metastatic melanoma or metastatic ovarian cancer. The result, in every patient who had received a particular kind of vaccine, was widespread death of cancer cells and an increase in the number of immune system cells within the tumors - evidence of a potent immune system attack.
"This study makes a strong case that combined immunotherapy - consisting of a vaccine and antibodies - can elicit a potent immune response to some types of tumors in patients," said the study's senior author, Glenn Dranoff, M.D., of Dana-Farber, which is a principal teaching affiliate of Harvard Medical School.
The technique was inspired by the laboratory work of study co-author James P. Allison, a UC Berkeley professor of immunology, director of the campus's Cancer Research Laboratory and a Howard Hughes Medical Institute investigator. He and his colleagues discovered that a protein called CTLA-4, an antigen found on T cells, restrains the immune system from attacking cancer cells. In a series of laboratory and animal experiments, Allison's team showed that combining a cancer vaccine with an antibody able to block CTLA-4 resulted in an especially potent immune attack on tumors.
"Glenn got results in the first three or four patients, just like we got in mice," said Allison, a member of UC Berkeley's Health Sciences Initiative, which fosters collaboration among researchers in the biological and physical sciences. "There is a lot of excitement about this because you usually don't see any signs of drug activity in these early, Phase I trials, which are designed to look only at a drug's safety."
On the basis of Allison's findings, Dranoff and his colleagues launched a Phase I clinical trial of the technique in a small group of patients. Because animal experiments had indicated that giving MDX-CTLA4 in combination with a vaccine might prompt the immune system to attack some normal cells, the researchers decided to give the antibody to patients who had already been vaccinated.
Seven of the study participants had metastatic melanoma, a potentially fatal cancer that originates in skin cells, and two had metastatic ovarian cancer. In all three melanoma patients who had been treated with one form of vaccine, tumors showed extensive signs of cell death and were saturated with large numbers of tumor-fighting immune cells. The same results were seen in the two ovarian cancer patients who had been treated with the same type of vaccine. The vaccine is created by loading tumor cells with a gene called GM-CSF that alerts the immune system to the tumors' presence, prompting an anti-tumor attack.
Of the four melanoma patients who had received a different type of vaccine based on melanoma antigens, none experienced a similar benefit, the researchers found.
While none of the study participants had serious reactions to the antibody itself, some of the melanoma patients developed a mild immune reaction against normal skin cells called melanocytes, but it was not a dangerous side effect.
"In most of the patients, there were clear signs of anti-tumor activity, in that there was invasion by immune cells that you wouldn't have seen otherwise," UC Berkeley's Allison said. "The autoimmune symptoms are a sign of that, too. It has been known that progressive depigmentation syndrome, or vitiligo, is a very good sign in patients with metastatic melanoma. These patients have a very good prognosis."
Previous clinical trials have shown that vaccines can be at least temporarily effective in treating metastatic melanoma and ovarian cancer, but most patients eventually succumb to their diseases. One of the reasons for this may be that the CTLA-4 molecule gradually weakens the immune system's ability to recognize and respond to tumor cells.
"By blockading CTLA-4 with antibodies, we had hoped to strengthen the immune response produced by cancer vaccines," remarked Dranoff, who is also an associate professor of medicine at Harvard Medical School and a Leukemia and Lymphoma Society clinical scholar. "Work in the laboratory and in animal models suggested that this approach could be effective. The new study offers the first evidence that the technique has promise in human patients, although much more study will be needed to demonstrate that this is the case."
Other Phase I trials of the CTLA-4 antibody therapy are underway against prostate cancer and melanoma. They are being sponsored by Medarex, Inc., a New Jersey-based pharmaceutical company that makes antibody-based therapeutics and that has licensed the patented therapy from UC Berkeley and created the human antibody used in the study. Early results have encouraged other cancer researchers to add CTLA-4 therapy to treatment protocols for other types of tumors.
"The beauty of this, and the reason we stared working on this eight years ago, is that it potentially enhances the effectiveness of many therapies, whether immunological approaches, like tumor cell vaccines, or chemotherapeutic drugs or radiation," said Allison, who consults for Medarex on its drug trials. "Plus, in theory, it can be used against many types of cancer. It offers considerable promise, I think, in melanoma, prostate, ovarian, testicular and breast cancer.
"The implications are huge now that it's proven to be safe and has some effectiveness," he said. "It's a potential drug for human cancer now, not just a hypothesis based on mouse work."
The study's lead author is Stephen Hodi, M.D., of Dana-Farber and Brigham and Women's Hospital. Other co-authors are from Massachusetts General Hospital, Massachusetts Eye and Ear Infirmary, Harvard Medical School and Medarex.
Funding for the research was provided by the Berlex Oncology Foundation, National Institutes of Health, the Leukemia and Lymphoma Society, the Cancer Research Institute, and Medarex.

