UC Berkeley Press Release
Engineers create lab-on-a-chip using fluorescent dye to detect toxins
BERKELEY – Engineers at the University of California, Berkeley, have taken a common piece of tabletop lab equipment used to detect toxic substances and biochemical agents and mini-sized it to a device that fits on a tiny computer chip that could be perfect as a low-cost tool in the field.
The researchers said that those most likely to benefit from the achievement, which involves a unique laser-processing technique, are law enforcement or environmental pollution investigators, military medics or anyone who may need a cheap, portable device that can identify the presence of specific toxins or DNA on site rather than in a laboratory.
|
"Imagine if you needed to quickly determine whether a soldier in a battlefield had been exposed to a biochemical agent," said J. Alex Chediak, a UC Berkeley graduate student in materials science and engineering and lead author of the paper. "You don't have time to take a sample of his blood back to the lab and wait for results. This device could be developed into something medics use in the field to get answers in a matter of minutes, not hours."
UC Berkeley has recently filed a provisional patent application on the new device, which is described in the March 1 issue of Sensors and Actuators A. The National Science Foundation supported the research, led by Timothy Sands, a professor of engineering at Purdue University who conducted the work while he was a professor of materials science and engineering at UC Berkeley.
The lab-on-a-chip device uses fluorescence microscopy to signal the presence of target molecules in a sample. If molecules of a specific substance - such as an avian flu virus or anthrax - are present in the sample, the dye tags them. When light within a certain wavelength range hits a specific dye, the dye becomes "excited" and emits its own light, usually at a higher wavelength.
For example, a dye that is excited by blue light at about 490 nanometers emits a green light in a range of 510-530 nanometers. The more tagged molecules there are in a sample, the stronger the green signal. "One major difficulty is that only a small amount of the incoming light actually results in excitation and subsequent dye emission," said Chediak. "That's why it's so important to have a filter, especially if you want to measure trace concentrations."
Currently, machines used in fluorescence microscopy take up as much space as a desktop computer and cost upwards of $100,000. The elements needed in the system include a laser light source, a holding platform where a sample is placed, a filter that blocks unwanted light and a detector that senses light at specified wavelengths.
"We have developed a way of putting all of the system's critical components together on one wafer using a 'laser lift-off' technique we pioneered," said Sands, principal investigator of the research team. Sands explained that laser lift-off is a technique that allows very thin films of materials to be transferred from one substrate to another.
Such a transfer was performed to place the light source, a thin layer of gallium nitride, onto the silicon substrate. The atomic dimensions of the film and substrate need to match for the film to grow properly. Silicon was not a suitable match for the light-emitting layer, so the gallium nitride layer was grown on a sapphire crystal, and transferred to a silicon chip.
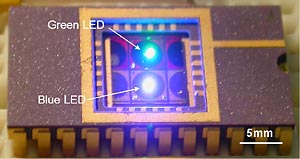
The entire chip is 5 square millimeters and 1.5 millimeters thick. Because chips can be mass-produced, the cost of manufacturing each unit could eventually drop to a few dollars. "We have the opportunity to utilize the tremendous advances in the semiconductor industry, in particular scaling and batch fabrication, to simultaneously fabricate hundreds of miniature on-chip laboratories," said Chediak.
Samples to be analyzed are held in a disposable microfluidic channel system that is placed on top of the chip. When the researchers compared the performance of the chip with that of a commercial filter, they found that both were equally effective at absorbing blue light, but that a commercial filter transmitted more green light.
"Even though the performance of the bench-top unit exceeded that of the microchip, the chip's small size, low cost and ease of production make it an attractive alternative for field use," said Zhongsheng Luo, a UC Berkeley graduate student in materials science and engineering and co-author of the paper.
The researchers have recently added a green LED with a matching filter onto the same chip, and presented the results of the two-color system at a scientific conference in June. "Having two color sources allows researchers to tag the sample with two dyes," said Sands. "One dye serves as a control to calibrate the measurements, and the other color is for actually detecting the molecule you're trying to find."
They are now in the process of comparing the dual-colored chip with bench-top instruments.
"Fluorescence detection microsystems may not entirely replace their larger counterparts, but they could eventually allow testing for chemicals and biological toxins in places where a large unit is now impractical," said Chediak. "That would be a valuable contribution to the biomedical field, benefiting both military and civilian communities. Processes now performed only in special laboratories may someday be possible in every home."
Other UC Berkeley co-authors of the paper are Luke P. Lee, assistant professor of bioengineering; Nathan Cheung, professor of electrical engineering and computer sciences; and Jeonggi Seo, graduate student in applied science and technology.