UC Berkeley Press Release
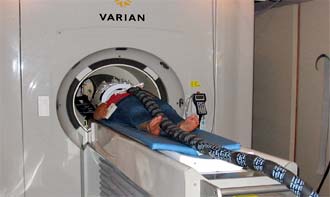 |
A student undergoing
a sniffing experiment inside a functional magnetic resonance
imager. The tube carries air and odors to the subject while
the fMRI images the brain and highlights areas of intense
activity. (Noam Sobel/UC Berkeley. |
How the brain tunes out odors
BERKELEY – Immersed as we are in a sea of smells, how is it we're not continually overwhelmed with fair or foul odors until we actively inhale a rose or sniff the milk for a hint of sourness?
University of California, Berkeley, neuroscientists have scanned the brains of people sniffing odors and found an answer.
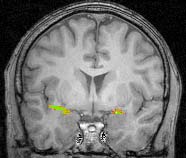 A composite fMRI scan of the brains of 10 subjects undergoing random sniffing trials, where left is the left side of the brain. The piriform cortex is lit up with activity. The temporal (outer) portion of the cortex processes odors and is active whether or not we are paying attention to the smells around us. The frontal (inner) portion and tubercle are most active when we pay attention to the odors. (Christina Zelano, Noam Sobel/UC Berkeley) |
It turns out that the brain is detecting and processing all the odors around us, but a particular area of the brain actively tunes this out unless the odor reaches a high level, such as when we walk into a cloud of cloying perfume or step in dog poop.
When we want to sniff for odors, however, the brain releases the block and begins to pay attention to the smells around us. It even tunes in very precisely to specific smells, allowing us, for instance, to search for a hint of blackberry in a glass of zinfandel.
"We've identified a novel brain mechanism that functions as a gate for information, enabling our brain to focus on what our nose is telling us when we want it to, and more importantly, ignore it when we choose to," said Noam Sobel, assistant professor in the Helen Wills Neuroscience Institute and in the Department of Psychology at UC Berkeley.
The selective nature of our sense of smell fits with an emerging understanding of the other senses, including vision, hearing and touch. In all these cases, our perceptions are affected by whether the brain is paying attention or not.
"People tend to think of sensory processing as something passive. They think of the sensory animal, be it a human or whatever, sitting there and having stimuli thrown at it," he added. "But that's not at all true -- sensation is a very active process. Mammals evolved to actively seek out sensory information, and this is a very, very strong demonstration of that."
Sobel and his colleagues, including UC Berkeley biophysics graduate student Christina Zelano, the lead author, reported their results this week in the online version of Nature Neuroscience.
The areas involved in sensing odors were pinpointed by a functional magnetic resonance imager (fMRI) that captured pictures of the brain and its active areas as subjects sniffed both odorless and scented air. The most active areas during this experiment were in the piriform cortex, located behind the eyes at the bottom of the temporal lobe. The piriform cortex is a portion of the primary olfactory cortex -- the brain's grey matter that receives input from the olfactory bulb, which is the hub of information coming from the nose.
"When you're interested in what your nose is receiving, then you heighten the sensitivity in the frontal piriform cortex, but when you're not interested in knowing that, you actually inhibit activity in this area," said Sobel, who also is a member of the campus's Health Sciences Initiative.
The frontal piriform cortex is thus a gate that controls whether or not we pay attention to the smells registered by odor receptors in the nose. These odor signals still get to the brain, specifically the temporal piriform cortex, whether we're attentive or not.
"The temporal portion of the piriform cortex, however, gives you a big response every time you take a sniff," said Sobel. "It doesn't care if you're trying to detect an odor or just breathing in through your nose. Every time, you get this modulation of activity there."
Zelano, who studies the brain's response to odors in Sobel's laboratory, gathered 20 student volunteers for her experiment, which she conducted in the Wheeler Brain Imaging Center's 4 Tesla fMRI. Each subject was fitted with non-magnetic earphones and a device to deliver smells, called an olfactometer, developed by Sobel and coauthor Brad Johnson, a bioengineering graduate student.
In separate trials, subjects were presented with both odorless and scented air and told to sniff at the sound of a tone. When they were told the experiment was about sound detection, the frontal piriform cortex showed lowered activity, whether an odor was present or not, indicating that the attention to odors was being actively suppressed.
When told the experiment was about odors, however, the frontal piriform cortex became much more active, whether or not an odor was presented. Odors used included amyl acetate, which smells like bananas; phenyl ethyl alcohol, which smells like roses; propionic acid, which smells like vinegar; eugenol, which smells like cloves; and citral, which smells lemony.
The frontal piriform cortex, which is the attention gate, actually became active before the subjects sniffed, showing that the brain actually anticipates an odor, Sobel said.
"That shows you that in the olfactory cortex, the pattern of activity reflects what you intend on doing. If you intend to locate an odor, then you have more activity in that portion of the olfactory cortex," he said.
Zelano, Sobel and their colleagues plan to continue their fMRI studies to determine which portions of the brain are involved in very specific tuning of perception to a particular smell.
Coauthors of the paper include postdoctoral fellow Moustafa Bensafi, graduate students Joel Mainland, Elizabeth Bremner and Christina Telles, and senior scientist Rehan Khan, all of the Helen Wills Neuroscience Institute; biophysics graduate student Jess Porter; and bioengineering graduate student Brad Johnson.
The work was supported by a grant from the National Institute of Deafness and Communication Disorders of the National Institutes of Health. Zelano is supported by a National Science Foundation predoctoral fellowship.

