UC Berkeley Press Release
Scientists discover that three overlapping signals in embryo help get the backbone right
BERKELEY – A major step in the development of the vertebrate embryo - the establishment of a back that morphs into a brain, spinal cord and muscles - turns out to be so important that the body uses at least three signals to make sure it happens properly.
The discovery, reported this month in the journal Developmental Cell by researchers at the University of California, Berkeley, finally explains an 80-year-old observation that revolutionized the way biologists think about embryonic and fetal development and set the stage for the stem cell debate.
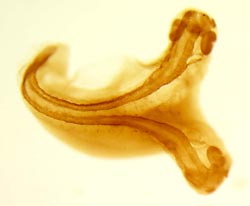 A Xenopus tropicalis tadpole that twinned after a Spemann's organizer from another embryo was transplanted into its belly. The backbone and developing brain are easily visible. (Photo by Harland lab/UC Berkeley) |
That 1924 observation in newts by Hans Spemann and Hilde Mangold earned Spemann the Nobel Prize in 1935 and generated the notion that embryonic cells don't know what they'll become until they get the proper signal. This concept is at the root of today's excitement over embryonic stem cells, which are basically naive cells that, theoretically, can be stimulated to become any tissue of the body.
In fact, the proteins normally used by the embryo have recently been put to use in embryonic stem cell work. Noggin, one of the proteins isolated by the UC Berkeley research group, has been used in cultures to maintain the growth of neural stem cells.
The new UC Berkeley experiments, on frogs, show that some steps in early embryonic development are so critical that many overlapping signals are needed to ensure that cells go down the right path. The formation of the back and belly is a milestone for frogs as well as for humans and other vertebrates, occurring as it does at the beginning of the process of gastrulation, which sees front and back, head and tail, left and right established and the first appearance of a recognizable body plan. If this step fails, the embryo eventually dies.
"Gastrulation and the process of defining your back-belly axis is such an important step that you actually have multiple proteins being expressed there, just in case one of them fails, the others can compensate," said lead author Mustafa Khokha (pronounced KO' ka), a post-doctoral fellow at UC Berkeley and an attending physician in pediatric critical care at UC San Francisco. "Redundancy is designed to make the embryo robust, so as to avoid birth defects."
The three proteins identified in the team's report are among at least five that block other signals to allow the back, or dorsal, structures to form. Thirteen years ago, Richard Harland, professor and chair of the Department of Molecular and Cell Biology at UC Berkeley, showed that injecting one of these factors, noggin, into the belly of an embryo caused surrounding tissues to develop into structures normally found on the back. Despite repeated attempts, however, no one could show that blocking signals like noggin did the opposite. In fact, blocking noggin and the other known factors seemed to have relatively little effect on the embryo, raising doubts about the natural role of these proteins in early development.
Now, using very young embryos of the laboratory frog Xenopus tropicalis, Khokha, Harland and their UC Berkeley colleagues were able to block three of the five factors at once, and this time found dramatic changes in the embryo.
"When we removed these signals, all the tissues that form on the back of the embryo - brain, spinal cord and muscles - were lost," Khokha said. "Not only were back tissues lost, but belly tissues were greatly expanded - the whole embryo became repatterned, so it's more belly-like than it is back-like. So, these signals are necessary for the pattern to occur properly."
"We first found these signals in 1992, and since then, we've figured out how they work. But because there are so many of them, it's been difficult to really nail down that they are essential," Harland said. "We've had to knock down three of them to prove that they're essential."
In the 1920s, working at the University of Freiburg in Germany, Spemann and his student Mangold found that as the mass of cells in the embryo started to take form, a region in the embryo - what they called the "organizer" - seemed to be the source of signals to nearby cells, telling them to form back structures. What clinched their experiment was taking the organizer from one embryo - in their experiments, a newt - and implanting it into another.
"They actually produced a Siamese twin embryo," Khokha said. "The piece they cut out from the donor embryo and transplanted into the host embryo was able to redefine the structures in the host embryo to make back structures where the belly would normally form - to induce another head, brain and spinal cord. The tissue they transplanted sent signals to the host to create these new structures."
Since then, scientists have searched for the signaling factors secreted by Spemann's organizer. Dubbed bone morphogenetic protein (BMP) antagonists, these factors block a process that creates belly structures and clear the way for back development.
"It is really in the last 20 years that it has been possible to tease these apart cleanly and develop assays that are good enough to find the molecules involved in the normal process," Harland said.
Postdoctoral fellow Bill Smith and Harland found the first of these BMP antagonists, noggin, in 1992, confirming its activity by repeating Spemann and Mangold's experiment but injecting noggin instead of implanting an organizer from another embryo. They also discovered xnr3, and other researchers isolated three more factors - chordin, follistatin and cerberus. While Harland and his UC Berkeley colleagues found numerous roles for noggin at later stages of development, they were unable to prove that noggin was essential to brain and spinal cord development. Blocking any one of these five factors had only a minimal effect on the fate of the embryo.
Khokha and Harland decided to try blocking more than one, and chose to work in embryos of the frog Xenopus tropicalis, a close relative of the more common laboratory frog Xenopus laevis. X. tropicalis is easier to work with, because it, like humans, is diploid, that is, it has only two copies of each gene, instead of four, as in the tetraploid X. laevis. Lab specimens of X. tropicalis also have less genetic variation than outbred populations of X. laevis, and the genome of X. tropicalis has been sequenced. Harland is pioneering the use of X. tropicalis in genetic studies, creating numerous mutants that can be used to explore the role of various genes in development.
Using standard knock-out techniques, their team inactivated the function of these BMP antagonists in various combinations, finding the most dramatic effect by simultaneously knocking out noggin, follistatin, and chordin.
Harland noted that the signaling pathway they blocked is one of several developmental pathways proceeding at the same time in the embryo. While BMP antagonists allow the formation of the back and belly, another group of antagonists creates the head and tail, while a third sets up left and right.
"It's been interesting that what one thinks of embryologically as the dominant signaling center actually is a source of a cocktail of inhibitors," he said, "so inhibition is just as specific a signal in the embryo as is an activating signal."
Since they discovered noggin, Harland and his colleagues have shown that in later stages of development, this protein factor is critical in laying down cartilage to make joints, and even plays an important role after birth. Recently, he and Stanford University colleagues showed that noggin may be important in preventing the premature fusion of the bones in the skull, and thus may be critical to allowing the brain to grow larger after birth. All of these findings are from mice or amphibians, but the researchers say that the same is almost certain to be the case in humans.
"One of the things that's been nicely shown is that the organizer itself, while it was originally identified in newts, is conserved through all vertebrate evolution," Khokha said. "If you cut out a similar tissue and transplant it in a mouse, you also get the Siamese twin phenomenon. So, we expect it to also be true in humans."
Coauthors of the current paper with Khokha and Harland are undergraduate Joanna Yeh and postdoctoral fellow Timothy C. Grammer of the Department of Molecular and Cell Biology at UC Berkeley. The research was funded by the National Institutes of Health, the National Institute of Child Health and Development of NIH, and UC Berkeley's Center for Integrative Genomics.

