UC Berkeley Press Release
New research provides evidence that Rh proteins act as CO2 gas channels
BERKELEY – Labels marking bags of donated blood throughout the world contain information about the presence of a Rhesus (Rh) antigen, a protein found on the membranes of human red blood cells. Yet, despite the Rh protein's importance in blood transfusion and the problems it can cause between Rh negative mothers and their Rh positive fetuses, its biological role has remained largely unresolved since its discovery 65 years ago.
But a new study, led by biologists at the University of California, Berkeley, and due to be published June 22 in the journal Genetics, may help clarify the mystery by giving additional evidence that the Rh protein serves as a gas channel for carbon dioxide (CO2).
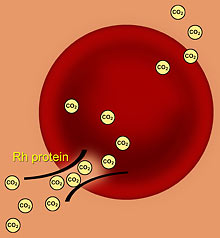 Rh proteins act as gas channels that help speed the transfer of carbon dioxide (CO2) in and out of red blood cells. CO2 can also pass through the cell membrane unaided (above right), but not quickly enough, said UC Berkeley researchers. (Image by Barbara Alonso) |
The study's conclusions, the researchers said, will lead to new directions in human and animal physiology research, as well as generate lively debate among biochemists and hematologists.
"This has implications for understanding how humans breathe, how we control the acidity (pH) of various fluids in our bodies and how our kidneys function, all of which rely upon movement of CO2 across cell membranes," said Sydney Kustu, professor of plant and microbial biology at UC Berkeley's College of Natural Resources and principal investigator of the study.
Kustu noted that scientists had long doubted the presence of protein channels for CO2 or any gases. This is because gases typically have no trouble crossing cell membranes unaided, so it was not suspected that the Rh protein would play such a role.
But among gases, carbon dioxide (CO2) and ammonia (NH3) are exceptional, the researchers explained. They both dissolve readily in water, which can slow their passage across oily membranes.
Recent evidence indicates that ammonium/methylammonium transporter (Amt) proteins act as gas channels for NH3. Unlike active transporters, channels allow multiple molecules of gas to move through at the same time, an important distinction for gases that need to move across membranes quickly.
Until a few years ago, scientists thought that Amt handled the charged ion for ammonia (NH4+), which is the major form found in water. They also believed Amt was an active transporter and used energy to move the ion molecules in or out of cells against a gradient.
Notably, Rh and Amt proteins are more closely related to each other than any other proteins. Because of this, many scientists have suggested that Rh proteins also function as active transporters for charged ammonium ions.
"Our research corrects that assumption by showing that Amt proteins are working with ammonia gas, and Rh proteins are working with carbon dioxide" said Kwang-Seo Kim, a research specialist at the Kustu Lab and lead author of the paper. "Other experiments that suggest that Rh proteins transport ammonium ion have involved cloning Rh genes into microorganisms or cells that do not have them naturally."
The researchers reached their conclusions by studying the humble green alga, Chlamydomonas reinhardtii, one of the few microorganisms known to have both Rh and Amt proteins. Amt proteins are widespread among microbes and plants, which use ammonia as the preferred source of nitrogen, a critical nutrient.
In contrast, Rh proteins are rare among microbes, yet common in vertebrates. Ammonia is toxic to vertebrates and, not surprisingly, Amt proteins are absent in this class of animals.
The researchers suggested that green algae have Rh proteins because they thrive in aquatic environments with high concentrations of CO2. Algae use sunlight energy to capture CO2 by photosynthesis.
Kustu and other researchers had previously shown that expression of the Rh gene in C. reinhardtii was high for cells grown in air supplemented with 3 percent CO2, about 100 times the concentration normally found in air. When the algae were grown in air, expression of the Rh gene was low.
They've also found that strains of C. reinhardtii in which the Rh protein was missing did not grow well in environments with high levels of carbon dioxide, suggesting that Rh was necessary for the algae's ability to benefit from high CO2 levels.
In this new study, Kustu and her research team isolated strains of C. reinhardtii in which an Amt gene was inactivated. They found a 90 percent reduction in uptake of the ammonia analogue methylamine in those mutated strains of green algae. In contrast, strains of the green algae in which the Rh gene was inactivated remained sensitive to methylamine and showed no deficiencies in the uptake of the ammonia analogue.
"This paper is the last piece in a puzzle about Amt and Rh proteins," said William Inwood, a research specialist in the Kustu Lab and the senior author of the study. "We've provided strong evidence that the substrate for the Amt protein is ammonia, while the substrate for the Rh protein is carbon dioxide within the same organism."
What do Rh proteins in green algae have to do with Rh proteins in humans? "It turns out that if you know the biochemical function of a protein, you know it," said Kustu. "A protein's biochemical function does not change from organism to organism."
Kustu also notes that the Amt channels are needed when ammonia is available in low concentrations, while the Rh channels kick into gear at high concentrations of carbon dioxide like those found in humans. The concentration of CO2 in human breath is as high as 5 percent.
The Rh protein's role in CO2 transport makes sense given its location on the surface of red blood cells, the researchers said. "Red blood cells need to transport CO2 from body tissue out through the capillaries of the lungs very quickly, and the Rh protein in blood cells is about speed," said Kustu.
Scientists have long taken an interest in the role of Rh protein in the shape and flexibility of red blood cells. The red cells of people who lack all Rh antigens, an extremely rare condition, are misshapen and easily ruptured. It is believed that the Rh protein helps to maintain the flexible, flattened shape of red blood cells. "This structural role is secondary to its original function as a gas channel for CO2," said Kustu. "It seems to be a newly evolving role that increases gas transport in red cells by increasing their surface area."
In addition to being present in red blood cells, Rh proteins are found in a variety of human organs including seminal vesicles and the kidney and brain. "Their location points to the multiple roles Rh proteins play in human physiology," said Kustu. "All this is to say that there is more to Rh proteins than previously thought, and they deserve more research. Studying them will lead in many interesting directions."
Other co-authors of the paper are Eithne Field, a former manager at the Kustu Lab at UC Berkeley; Natalie King, a post-doctoral researcher at the State University of New York at Buffalo's Department of Microbiology; and Takuro Yaoi, a scientist at Panomics, a research technology firm in Redwood City.
The research was funded by grants from the National Institutes of Health and from Syngenta's Torrey Mesa Research Institute, which closed in 2003.

