UC Berkeley Press Release
Brain hormone puts brakes on reproduction
BERKELEY – University of California, Berkeley, researchers have discovered a new actor in the mammalian reproductive system, a hormone that fills a role long suspected, but until now undetected.
The hormone, a small protein, or peptide, called gonadotropin-inhibitory hormone (GnIH), puts the brakes on reproduction by directly inhibiting the action of the central hormone of the reproductive system - gonadotropin releasing hormone (GnRH). GnRH stimulates the pituitary gland to activate the reproductive system, whereas GnIH appears to reduce the effects of GnRH stimulation.
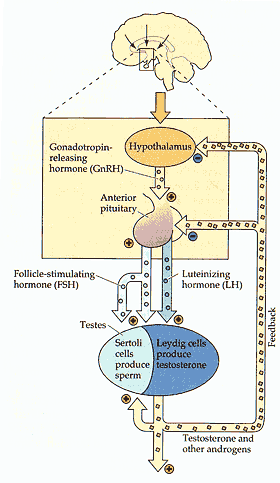 The reproductive system in males, which is regulated similarly in females, involves many feedback loops that regulate fertility much like a thermostat regulates temperature. The newly discovered hormone, gonadotropin-inhibitory hormone (GnIH), appears to be produced by the hypothalamus, where it seems to inhibit the production of gonadotropin-releasing hormone (GnRH), thereby putting a brake on reproduction at the very top of the reproductive axis. (Credit: Mark R. Rosenzweig, S. Marc Breedlove, and Neil V. Watson [2004]) |
Researchers have long sought inhibitors of pituitary gonadotropins, but many had come to believe that such a direct inhibitor was unlikely in the complex cast of hormones and factors in the reproductive system. The inhibiting or braking hormone may complement the "gas pedal" role played by another recently discovered hormone, kisspeptin, that stimulates GnRH.
The discovery in rats, mice and hamsters of this new system for regulating reproduction strongly suggests that the hormone plays a similar role in the reproductive systems of humans and other mammals. The human genome, in fact, contains a gene for GnIH.
If the new finding is mirrored in humans and other mammals, it would offer physicians another means of tweaking the reproductive system to fix problems ranging from infertility to precocious puberty, and also provide animal breeders with a new way to manipulate the productivity of livestock.
The findings by Kriegsfeld and colleagues are reported this week in the online early edition of Proceedings of the National Academy of Sciences.
The human reproductive system is regulated like a thermostat, with a number of hormones and factors produced along the "reproductive axis" acting via feedback loops to keep the body's hormones within the optimal range for fertility and successful mating. The head of the axis is the brain's GnRH-producing hypothalamus, which communicates via a blood portal with the anterior pituitary and stimulates production of hormones called gonadotropins, luteinizing hormone and follicle stimulating hormone.
These hormones are dumped into the bloodstream and make their way to the gonads, where in males they stimulate production of testosterone and the maturation of sperm. In females, the hormones stimulate production of estradiol, a sex steroid hormone and the body's main form of estrogen, and regulate ovulation, the production of fertile eggs.
Estradiol and testosterone, in turn, feed back on the pituitary to shut down production of pituitary hormones, establishing feedback that keeps the body's sex hormones on an even keel.
Estradiol also works higher in the brain, on the hypothalamus, to ramp down production of GnRH, but how this works has been a relative mystery. The new study provides an answer: Estradiol stimulates cells in the dorsomedial nucleus of the hypothalamus to produce GnIH, which appears to act directly on cells in the hypothalamus to turn off their production of GnRH.
"Here, we have a novel neural pathway mediating the regulatory actions of sex steroids," said Lance Kriegsfeld, UC Berkeley assistant professor of psychology.
"This is an example of the reproductive system being fine tuned," said George Bentley, UC Berkeley assistant professor of integrative biology. "We know a lot about the gross regulation of the reproductive system, but fine tuning hasn't been well understood at all."
GnIH was discovered five years ago in quail by Japanese researchers led by Kazuyoshi Tsutsui, a professor on the faculty of integrated arts and sciences at Hiroshima University. The discovery supplied one of the last remaining pieces of the bird's hormone system that controls reproduction. GnIH seemed to be the missing antagonist that switches off pituitary gonadotropins, and work by Tsutsui and Bentley in quail and white-crowned sparrows confirmed its role in turning down production of GnRH and thus switching off the gonads.
While Bentley collaborated with Tsutsui to determine how GnIH works in birds, the two also teamed up with Kriegsfeld to explore the implications in mammals. Kriegsfeld began collaborating with the group as a postdoctoral fellow working with Rae Silver, Kaplan Professor of Natural and Physical Sciences at Barnard College, and professor of psychology at Columbia University, and has continued this research in his own lab as an assistant professor. Using fluorescent antibodies to GnIH, they were able to locate where in the brain the hormone is made: in the nerves of the dorsomedial hypothalamus. The axons of these nerves project to numerous areas of the brain where nerve cells produce GnRH, and staining showed that these axons contacted GnRH-producing cells, suggesting direct effects.
As in birds, GnIH also rapidly inhibits production of luteinizing hormone by the pituitary, they showed. Along with the fact that the cells containing GnIH also have receptors for estrogen-like compounds such as estradiol, the combined evidence suggests that GnIH is a direct inhibitor of GnRH.
Because the brain cells producing and secreting GnIH send their axons into many areas of the brain, GnIH may have other effects on the brain, too.
"Though we don't know in mammals where the receptors are for GnIH, it looks like the hormone produces multiple effects in the brain," Bentley said. "In birds, the hormone not only affects reproductive hormones, but also sexual behavior in females, such as readiness to copulate."
Interestingly, the neurons producing GnIH are in an area of the brain, the dorsomedial nucleus of the hypothalamus, that coordinates and integrates information from external stimuli, the senses and from motivational and emotional inputs.
"It's likely serving lots of functions relating to motivated behaviors, such as reproduction or feeding," Kriegsfeld said.
Kriegsfeld and Bentley plan to continue their investigation of the role of GnIH in birds and mammals, with the assistance of postdoctoral fellow Takayoshi Ubuka, formerly of Tsutsui's laboratory in Japan, and Tsutsui's Hiroshima University colleagues, Kazuhiko Inoue and Kazuyoshi Ukena. Also coauthors of the PNAS paper were Dan Feng Mei and Rae Silver of the Barnard College and Columbia University Departments of Psychology, and Alex O. Mason of the UC Berkeley Department of Psychology. Kriegsfeld, Bentley and Mason also are members of UC Berkeley's Helen Wills Neuroscience Institute. Tsutsui and Ukena also are affiliated with the Core Research for Evolutional Science and Technology at Tokyo's Japan Science and Technology Corporation. Rae Silver is also Professor in the Department of Anatomy and Cell Biology at Columbia University Health Sciences.
The work was supported by the National Institute of Neurological Disorders and Stroke and the National Institute of Mental Health.

