UC Berkeley Press Release
Stretching bone marrow stem cells pushes them towards becoming blood vessels
|
BERKELEY – When stretched, a type of adult stem cell taken from bone marrow can be nudged towards becoming the type of tissue found in blood vessels, according to a new study by bioengineers at the University of California, Berkeley.
Researchers placed mesenchymal stem cells, extracted from bone marrow, onto a silicone membrane that was stretched longitudinally once every second. It was a cellular workout routine that helped point the stem cell in the direction of becoming the smooth muscle tissue of vascular walls.
The findings, published today (Monday, Oct. 23) in the online early edition of the Proceedings of the National Academy of Sciences, highlight the importance of mechanical forces in stem cell differentiation.
Mesenchymal stem cells have the ability to turn into different types of connective tissue including bone, cartilage and muscle. Embryonic stem cells have the advantage of being able to turn into any kind of body tissue and of being easier to work with in the lab, though that flexibility comes with controversy and ethical questions not found in research on adult stem cells.
But research on both types of stem cells holds the promise of treatment for diseased or damaged body parts. Experiments in stem cell differentiation, however, have traditionally relied upon chemical signals to prompt this transformation into the desired cell type.
| In research related to the study published in the Proceedings of the National Academy of Sciences (PNAS), members of Song Li's lab have been developing vascular grafts from mesenchymal stem cells positioned in a polymer tube. The hope is that once implanted in the body, the polymer will dissolve, leaving behind stem cells that have developed into a working blood vessel. On Thursday, Oct. 19, Craig Hashi, a graduate student in Li's lab and co-author of the PNAS paper, and Yiqian Zhu, a graduate student in bioengineering, won the graduate prize in the Collegiate Inventors Competition for their work on the vascular graft. The competition is an annual program of the National Inventors Hall of Fame Foundation, and it came with a $15,000 prize for Hashi and Zhu, and a $3,000 award for Li. |
Song Li, UC Berkeley associate professor of bioengineering and principal investigator of the study, heads one of the leading research groups in the country investigating the role of a stem cell's physical environment on its development.
"The mechanical effects on the body are well known. A good example is when astronauts in space experience a loss of bone mass because there is no gravity," said Li, who is a member of the California Institute for Quantitative Biomedical Research (QB3), a partnership between the state of California, industry, and the UC campuses at San Francisco, Berkeley and Santa Cruz. "We are now extending this concept to the cellular level by showing that mechanical stimulation can impact stem cell differentiation."
In an effort to better understand the factors that affect the eventual fate of mesenchymal stem cells, the researchers designed the experiment to simulate the physical forces a cell would encounter if it were to become a blood vessel.
Kyle Kurpinski, a UC Berkeley graduate student in bioengineering and lead author of the study, noted that in previous studies on the effects of mechanical strain, cells were stretched in all directions. However, he pointed out that cells in the walls of a blood vessel are pulled in a circumferential direction, or sideways if the vessel walls are laid flat. This new study is the first to look at the effects of such uniaxial strain on stem cell differentiation.
A one-stretch-a-second pace was chosen to correspond to a typical adult pulse rate, said Kurpinski. The researchers placed a single layer of mesenchymal stem cells onto a membrane with microgrooves to resemble the patterns formed in blood vessels by collagen fibers.
The microgrooves were aligned either perpendicular or parallel to the axis of strain, and some of the cells were stretched while others were not. The researchers also looked at the effects of stretching stem cells on a smooth membrane with no microgrooves.
"It is hypothesized that cells aligned in the microgrooves actually feel the strain of the pull more than if they were on a smooth surface," said Kurpinski.
After two days of this cellular exercise regimen, the researchers found a significant increase in the expression of a group of genes that control tensile strength compared with cells that were not stretched. Tensile strength is important for tissue that must withstand pulling forces, such as in vascular walls. Specifically, there was an increased level of calponin 1, a genetic marker for smooth muscles.
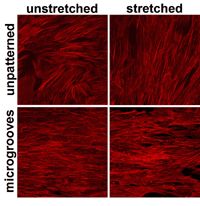 The cytoskeleton of mesenchymal stem cells shows the orientation of stem cells on micropatterned membranes. On unpatterned membranes, stem cells naturally orient randomly, but realign perpendicularly to the axis of stretch when repeatedly stretched. However, on membranes with micropatterned grooves, stem cells align with the grooves and remain aligned even when stretched. Note: direction of stretch is left to right, and the orientation of microgrooves is left to right. (Courtesy of Kyle Kurpinski, UC Berkeley) |
At the same time, expression of a group of genes associated with compression-bearing tissue, such as cartilage and bone, decreased. "For cartilage and bone, particularly at the joints, cells experience compression forces," said Li. "Stem cells seem to know the type of tissue they are supposed to become by the type of mechanical strain they are subjected to."
As for cell positioning, the researchers found that without the microgrooves, the stem cells would align themselves perpendicularly to the direction of the stretch. In contrast, when stretched on a membrane with microgrooves parallel to the axis of strain, the stem cells aligned themselves along the grooves.
They found that the perpendicular orientation significantly diminished the expression of genes for tensile strength. Researchers also saw a slight increase in cell proliferation when cells were aligned parallel to the axis of strain.
The findings indicate that the stem cells were well on their way to becoming smooth muscle tissue, although they didn't quite get there.
"The potential is there," said Li. "We are halfway done. To completely achieve the efficient guidance of cell differentiation in a lab, we will likely need a combination of chemical and mechanical factors."
"We're definitely a major step closer to developing a process of tissue engineering that could one day have clinical applications," added Kurpinski.
Other co-authors of the study are Julia Chu, a research associate in Li's lab, and Craig Hashi, a UC Berkeley graduate student in bioengineering.
The National Institutes of Health helped support this study.