UC Berkeley Press Release
Old muscle gets new pep in UC Berkeley stem cell study
BERKELEY – Old muscle got a shot of youthful vigor in a stem cell experiment by bioengineers at the University of California, Berkeley, setting the path for research on new treatments for age-related degenerative conditions such as muscle atrophy or Alzheimer's and Parkinson's diseases.
In a new study published June 15 in an advanced online issue of the journal Nature, researchers identified two key regulatory pathways that control how well adult stem cells repair and replace damaged tissue. They then tweaked how those stem cells reacted to those biochemical signals to revive the ability of muscle tissue in old mice to repair itself nearly as well as the muscle in the mice's much younger counterparts.
Irina Conboy, an assistant professor of bioengineering and an investigator at the Berkeley Stem Cell Center and at the California Institute for Quantitative Biosciences (QB3), led the research team conducting this study.
Because the findings relate to adult stem cells that reside in existing tissue, this approach to rejuvenating degenerating muscle eliminates the ethical and medical complications associated with transplanting tissues grown from embryonic stem cells.
"We are one step closer to having a point of intervention where we can rejuvenate the body's own stem cells so we don't have to suffer from some of the debilitating diseases associated with aging," said the study's lead author, Morgan Carlson, a recent Ph.D. graduate of Conboy's lab.
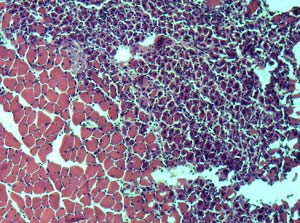 On the right side of this muscle tissue from a young mouse are healthy, new cells created to replace damaged tissue. This ability to regenerate new cells diminishes with age. (Photos courtesy of Morgan Carlson and Irina Conboy, UC Berkeley) |
"We don't realize it, but as we grow our bodies are constantly being remodeled," said Conboy. "We are constantly falling apart, but we don't notice it much when we're young because we're always being restored. As we age, our stem cells are prevented, through chemical signals, from doing their jobs."
The good news, the researchers said, is that the stem cells in old tissue are still ready and able to perform their regenerative function if they receive the appropriate chemical signals. Studies have shown that when old tissue is placed in an environment of young blood, the stem cells behave as if they are young again.
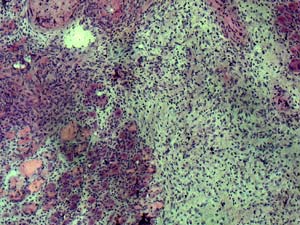 As muscle tissue ages, it loses its ability to adequately repair itself from damage. Instead of creating healthy, new cells to replace damaged ones, the old muscle tissue is left with fibroblasts and scar tissue, as shown here. |
Adult stem cells have a receptor called Notch that, when activated, tells them that it is time to grow and divide, the researchers said. But stem cells also have a receptor for the protein TGF-beta that sets off a chain reaction activating the molecule pSmad3 and ultimately producing cyclin-dependent kinase (CDK) inhibitors, which regulate the cell's ability to divide.
"Interestingly, activated Notch competes with activated pSmad3 for binding to the regulatory regions of the same CDK inhibitors in the stem cell," said Conboy. "We found that Notch is capable of physically kicking off pSmad3 from the promoters for the CDK inhibitors within the stem cell's nucleus, which tells us that a precise manipulation of the balance of these pathways would allow the ability to control stem cell responses."
 In this muscle tissue from an old mouse, UC Berkeley researchers manipulated the biochemical response of adult stem cells in the tissue, and the muscle was able to repair itself from damage almost as well as muscle from young mice. |
Aging and the inevitable march towards death are, in part, due to the progressive decline of Notch and the increased levels of TGF-beta, producing a one-two punch to the stem cell's capacity to effectively rebuild the body, the researchers said.
"What we discovered is the interplay between two pathways - one an aging pathway, and the other a youthful pathway," said Conboy.
But what would happen if researchers blocked the adult stem cells in old tissues from reacting to those TGF-beta signals? The researchers put that question to the test in a living organism by comparing the muscle regeneration capacity of old, 2-year-old mice, comparable in age to a 75- to 80-year-old human, with that of 2-month-old mice, similar in age to a 20- to 25-year-old human.
For a group of the old mice, the researchers disabled the "aging pathway" that tells stem cells to stop dividing by using an established method of RNA interference that reduced levels of pSmad3. The researchers then examined the muscle of the different groups of mice one to five days after injury to compare how well the tissue repaired itself.
As expected, the researchers found that muscle tissue in the young mice easily replaced damaged cells with new, healthy cells. In contrast, the areas of damaged muscle in the control group of old mice were characterized by fibroblasts and scar tissue.
However, muscles in the old mice whose stem cell "aging pathway" had been dampened showed levels of cellular regeneration that were comparable to their much younger peers, and that were 3 to 4 times greater than those of the group of "untreated" old mice.
The researchers cautioned that shutting down the TGF-beta/pSmad3 pathway altogether by turning off the gene that controls it could lead to many health problems. The ability to suppress cell division is critical in controlling the development of tumors, for instance.
"When we are young, there is an optimal balance between Notch and TGF-beta," said Conboy. "We need to find out what the levels of these chemicals are in the young so we can calibrate the system when we're older. If we can do that, we could rejuvenate tissue repair for a very long time."
The researchers also warn against interpreting this research as the cure-all for aging.
"We're not at a point where we're ready to inject ourselves with TGF-beta antibodies and call it a day," said Carlson. "There are multiple mechanisms involved in how our body functions. We know that TGF-beta is involved in one aspect of aging, but we don't know where it fits in the global scheme of aging."
In addition to their work on adult stem cells, Carlson and Conboy have also discovered that human embryonic stem cells can actually neutralize the effects of aging. Conboy received funding last year from the California Institute for Regenerative Medicine (CIRM) to pursue this line of research.
Michael Hsu, a former UC Berkeley postdoctoral researcher in bioengineering, also co-authored this paper.
This study was primarily supported by the National Institutes of Health and The Ellison Medical Foundation, with additional funds from a pre-doctoral training grant from CIRM.

