Drugs may prevent epilepsy & seizures after brain injury
| 14 July 2009
BERKELEY — Drugs that block a growth factor receptor on brain cells may prevent epilepsy after brain damage, according to a new study appearing in the July 15 issue of The Journal of Neuroscience.
Daniela Kaufer, an assistant professor of integrative biology at the University of California, Berkeley, graduate student Luisa P. Cacheaux, and their Israeli colleagues, graduate student Yaron David and neurosurgeon Alon Friedman, found that they could prevent the brain changes leading to epilepsy in rats by treating the animals with a drug that blocks transforming growth factor-beta (TGF-beta) receptors.
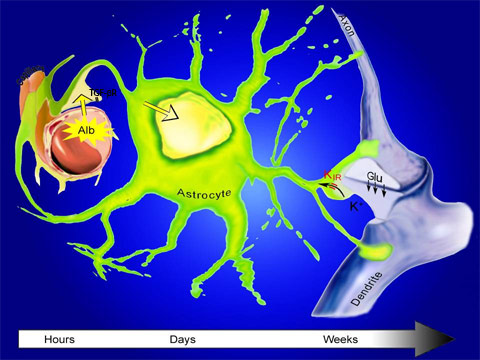 The blood-brain barrier lining the capillaries often breaks after a brain injury, allowing small amounts of blood to leak into the brain. Albumin (Alb) in the blood serum binds to the TGF-beta receptor (TGF-βR) on astrocytes, often called glial cells, triggering a host of changes. One of these prevents astrocytes from mopping up the two neurotransmitters, glutamate (Glu) and potassium ions (K). As a result, these neurotransmitters flood the synapses between nerve cells, where the axon of one neuron touches the dendrite of another neuron, causing a constant excitation that can destroy the nerve cell. These changes take place over a period of days, providing a large window for delivery of drugs to block TGF-beta and prevent such changes. (Dr. Dominik Zumsteg/Zurich University Hospital)
The blood-brain barrier lining the capillaries often breaks after a brain injury, allowing small amounts of blood to leak into the brain. Albumin (Alb) in the blood serum binds to the TGF-beta receptor (TGF-βR) on astrocytes, often called glial cells, triggering a host of changes. One of these prevents astrocytes from mopping up the two neurotransmitters, glutamate (Glu) and potassium ions (K). As a result, these neurotransmitters flood the synapses between nerve cells, where the axon of one neuron touches the dendrite of another neuron, causing a constant excitation that can destroy the nerve cell. These changes take place over a period of days, providing a large window for delivery of drugs to block TGF-beta and prevent such changes. (Dr. Dominik Zumsteg/Zurich University Hospital)While seizures can take weeks to show up in rats, for the current paper, the researchers followed the rats for only four days after brain injury and treatment with TGF-beta blockers. Nevertheless, preliminary EEG studies of the rats' brains indicated that most animals remained seizure-free after a month.
If the findings are confirmed in humans, a TGF-beta blocker may prevent many cases of epilepsy in accident victims or Iraqi war GIs who are victims of roadside bombs. Because of better medical care, many soldiers now survive severe traumatic brain injuries, yet those with severe head injuries are thought to have a 25 to 50 percent chance of eventually developing epileptic seizures. No treatment exists to prevent the development of epilepsy. Once epilepsy develops, drugs are the only option, and even those fail to control seizures in 30 percent of cases.
Because seizures develop weeks to years after an injury, there is a large window of opportunity in which patients could be treated with drugs to prevent the development of seizures, Kaufer said.
"The idea is to identify the brain injury patients that are very susceptible to epilepsy development - which may be possible to achieve using brain imaging - and then treat only those, not everybody, with a pretty benign drug that blocks the growth factors," she said. "At least in the rats, that works now."
The results are the culmination of more than 14 years of research to explore the hypothesis that trauma-induced epilepsy is caused by leakage of blood into the brain after injury, whether caused by trauma, brain tumors or infection, meningitis, or a hemorrhagic or ischemic stroke.
The idea originated with Friedman, who at the time was a physician in the Israeli army. Friedman, now an associate professor of physiology and neurosurgery at Israel's Ben-Gurion University of the Negev, hypothesized that breach of the blood brain barrier - a sheath of tightly joined cells that lines the capillaries in the brain to prevent intrusion of bacteria and potentially dangerous blood-borne molecules - somehow triggers events that destroy brain cells.
Friedman teamed up with Kaufer, then a graduate student at Hebrew University, on a series of experiments that has gradually provided support for the hypothesis and convinced many that this is a totally new and valuable way of looking at epilepsy. Over the years, their team systematically sifted through the components of the blood and, in 2007, reported that the main culprit in epileptogenesis seemed to be albumin, the main protein in blood serum.
In the current experiment, the researchers used serum albumin to trigger epileptogenesis in rats' brains and showed that albumin binds to TGF-beta receptors - there are two of them - and triggers the expression of a myriad of genes that are also turned on when the blood-brain barrier is breached by other means. The genes expressed involve not only the normal TGF-beta pathway, but also genes involved in inflammation and in reducing inhibition of neurons. The actual damage is thought to be caused by uninhibited firing of neurons, so called hyper-excitability, that can exhaust and kill the neurons. Neuron death alters the nerve network in the brain, leading to a reorganization of neurons that creates short-circuits that precipitate seizures.
"Epilepsy is neurons firing together in synchrony, which leads to a storm of electricity," Kaufer said. "The brain by itself has mechanisms - release of inhibitory signals through inhibitory neurotransmitters - to shut down the firing. In epilepsy, you don't get shutdown of firing, and it spirals out of control.
"Here we have shown the beginning stages of the hyper-excitable state when a lot of inhibitory genes are being down-regulated, so that you don't have as much inhibition. And then the synchrony begins."
The team triggered the same processes by squirting TGF-beta1 into the brain, and were able to block these genetic changes by treating the brain with drugs that block both TGF-beta receptor 1 and TGF-beta receptor 2.
Kaufer noted that TGF-beta blockers might also prevent further damage in those with persistent seizures - a condition known as status epilepticus - because these non-stop seizures also open the blood brain barrier.
Interestingly, the albumin initially seems to be activating receptors on astrocytes, not neurons. Astrocytes, also called glial cells, are a population of "support cells" in the brain that research is showing may play an important role in many disease processes.
"The astrocytes really work well as sponges for glutamate and potassium ions, controlling neuronal excitability," Kaufer said. "Signaling in the TGF-beta pathway changes the properties of astrocytes, so you get higher potassium and glutamate in the vicinity of neurons and hyper-excitability, which makes the neurons start firing together, you get synchronous activity developing, and epilepsy follows."
Friedman continues to monitor treated rats with an electroencephalograph (EEG) to see what percentage of the rats goes on to develop epileptic seizures. Friedman and his group in Ben-Gurion's Brain Imaging Research Center are developing new imaging tools that allow measuring the blood-brain barrier opening in humans with brain injuries.
"You can have somebody with no epileptic seizures, but the barrier is open for weeks and months after the trauma. We have initial evidence to suggest that these patients are much more susceptible to the development of epilepsy," Friedman said.
Kaufer and her lab colleagues continue to explore the role of blood-brain barrier breach in epilepsy, and the impact of stress on the brain.
Other coauthors of the paper are Sebastian Ivens, a psychiatry resident, and Uwe Heinemann, a neurophysiologist, from the Institute of Neurophysiology at Charité Universitätsmedizin in Berlin; Alexander J. Lakhter and Guy Bar-Klein of the Zlotowski Center for Neuroscience at Ben-Gurion University; and Michael Shapira, UC Berkeley assistant professor of integrative biology. Kaufer and Cacheaux are also affiliated with UC Berkeley's Helen Wills Neuroscience Institute.
The work was supported by the CURE Foundation, German National Science Foundation, Mary Elizabeth Rennie Epilepsy Foundation, Israel Science Foundation and United States-Israel Binational Science Foundation.

